
Our services
MAECON provides services to a wide range of stakeholders including MedTech, pharmaceutical industry, healthcare professionals and patients (organizations). These include clinical efficacy trials, consultancy, laboratory testing and educational courses on medication adherence. Read more by clicking on the stakeholder buttons on the right.
MAECON provides services to a wide range of stakeholders including MedTech, pharmaceutical industry, healthcare professionals and patients (organizations). These include clinical efficacy trials, consultancy, laboratory testing and educational courses on medication adherence. Read more by clicking on the stakeholder buttons below.
Our showcases
MAECON performs a variety of medication adherence related projects. These include studies on (pharmaco)epidemiology, usability and bioanalysis to randomized clinical trials of adherence enhancing interventions and digital devices. Further, educational, awareness and intervention development programs are internationally provided. A selection is showcased below.

Development of a real-world registry for digital inhalers (AURORA)
Digital inhalers are currently being assessed in clinical studies and some have recently been introduced on the market. Indeed, digital inhalers could help in management

Usability assessment of a novel smart blister
Recently, an electronic version of the Dosepak® (EDP) has been developed to monitor adherence to tablets, pills or capsules. It consists of a customizable blister

Development of a practical adherence toolkit (TAI Toolkit)
To be most effective, interventions focusing on enhancing adherence should be evidence-based and provided in a personalized manner. To identify personal issues with adherence in
Check our latest news
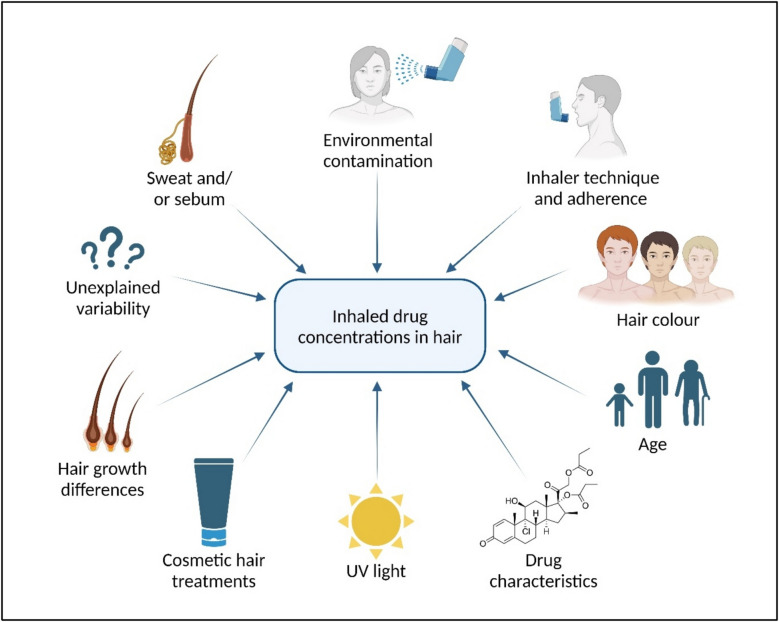
Novel method to assess long-term medication adherence in human scalp hair
MAECON has recently developed a novel analysis to detect exposure to drugs in human scalp hair. Given hair vessels are connected with the systemic blood circulation and hair grows around 1 cm per month, each centimeter of hair measured from the scalp, represents the average exposure. This way, using a

Winner national KNMP Innovation Prize!
On March 11, during the Dutch Spring congress of the Royal Dutch Pharmaceutical Association (KNMP), the project “Point-of-care FeNO measurement in the pharmacy”, with participation of MAECON’s founder Job van Boven from the UMCG, has won the KNMP Healthcare Innovation Prize! This Prize, worth €10,000, is annually awarded for advances

COST Action ENABLE successfully completed
End of 2024, European Commission funded COST Action ENABLE (European Network to Advance Best practices & technoLogy on medication adherencE, CA19132) has been successfully completed. This COST Action with 40 participation from 40 countries and led by MAECON, ran for the last 4 years and resulted in over 20 publications.
The team behind MAECON
MAECON works at the University Medical Center Groningen (UMCG) and/or the University of Groningen (UG). The members are independent senior researchers and represent a wide range of sub-specialisms related to medication adherence.
